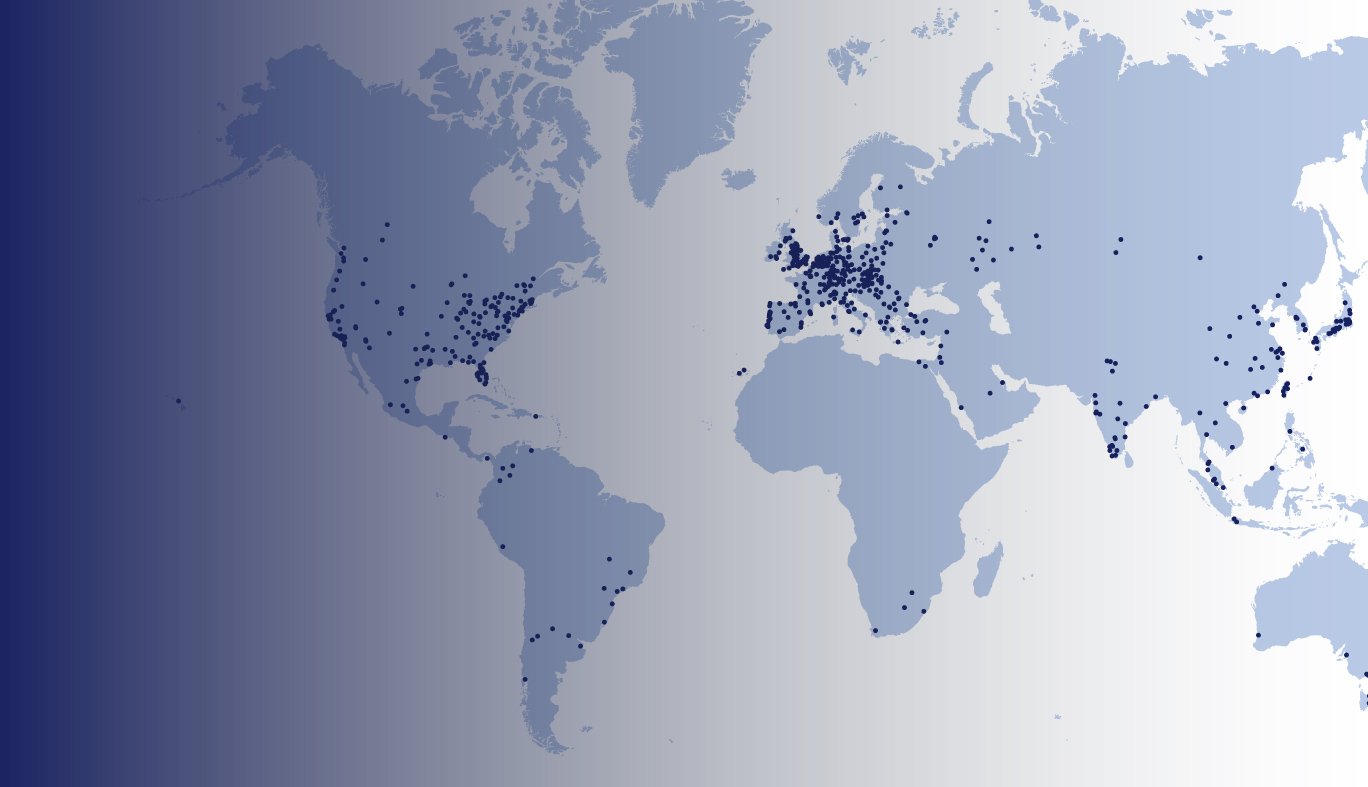
Our Reach
Klinitrial certifiers have delivered BCVA certifications in over 60 countries worldwide for 55 ophthalmic clinical trials.
Klinitrial maintains a live database that includes more than 1400 global clinical sites.
This valuable resource helps us to inform our sponsors with regards to successful site selection, expediting screening and subject enrollment. From our base in the UK, we deliver remote and on-site services worldwide to pharmaceutical companies and contract research organizations participating in ophthalmic clinical trials.
We have already provided over 4000 site certifications across these 1400 global sites.
Europe
We have certified 650 sites across 30 countries in Europe.
North America
We have certified 400 sites across North America.
Asia
We have certified 250 sites across 12 countries in Asia.
Australasia
We have certified over 30 sites in Australasia.

Work With Us
Through our comprehensive training and certification programs we are able to offer proven solutions for ophthalmic site data collection.
Our aim is to ensure conformity to study protocols thereby enabling reliable and repeatable results so that ophthalmic products can be successfully developed and approved based on precise measurements of visual acuity and investigative procedures.
All of our packages are individually designed to cater to the needs of our clients.





